Lambda Vectors
Vectors
Vector
Systems
Biology
Vectors
However, although 40% of the lambda genome is dispensible, virus particles containing less than 75% (and more than 105%) of the wild-type genome length (50KB) spontaneously fall apart. Therefore, viral genome packaging imposes a size constraint on the use of lambda as a cloning vector.
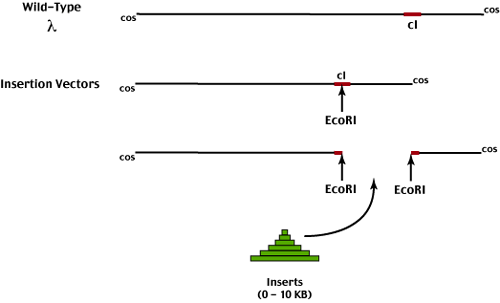
Insertion vectors are the simplest form of lambda cloning vectors.
The vector itself can be grown (therefore must contain at least 75% of the wild-type genome length).
Foreign DNA fragments are inserted into a unique restriction site in the vector genome. Packaging requirements thus limit insert fragment size to 0 - 10 KB - due to the limitations on viral genome size
(75% to 105% of the wild-type length = 50 KB)
In the construction of insertion vectors, 25% of the dispensible portion of the wild-type lambda genome was eliminated.
Insertion vectors must also contain a unique restriction site into which we can insert foreign DNA fragments. Since the lambda genome approximates a random 50 KB DNA sequence, we can expect most 6-mer restriction enzymes to cut several times (average frequency is 1/4000 bp). Removal of unwanted restriction sites involved mutagenesis of phage followed by selection for efficient growth on hosts carrying different restriction/modification systems. After multiple rounds of mutagenesis and selection, phage genomes were recovered that lacked unwanted restriction sites.
Since insertion vectors are large enough to be packaged into viable viruses, it is useful to have a
selection system that would allow us to identify recombinant phage containing foreign DNA inserts from non-recombinants containing only vector sequences.
For lambda insertion vectors, this selection system is based on the choice between lytic and lysogenic life styles. The phage genome choses between these alternatives based on the competition between positive and negative transcription factors expressed during the immediate early phase of the life cycle.
The negative transcription regulator is
the lambda cI gene product - the lambda repressor.
The lambda repressor acts to shut off lambda transcription.
Insertion of foreign DNA into the cI coding sequence therefore inactivates this negative regulator and forces all recombinant phage to replicate via the lytic cycle.
Non-recombinants can follow either the lytic or the lysogenic pathway.
As noted previously, E. coli containing a lambda provirus (a lambda lysogen) are immune to subsequent phage infection and so can grow in the presence of the virus.
This results in a 'cloudy plaque' morphology (cloudy appearance is due to the presence of lysogenic bacteria that continue to grow within the plaque).
Recombinant phage carrying a foreign DNA insert are unable to lysogenize (no negative regulator) and therefore have a 'clear plaque' morphology (no lysogenic hosts growing within the plaque).
While this visual morphology difference allows us to identify recombinant and non-recombinant plaques, it would be nice to have a selection system that allowed only recombinant phage to grow.
The identification of E coli Hfl strains (Hfl = High frequency lysogeny)
which affect the lambda lytic-lysogenic decision.
Instead of 99.9% of all phage infections following the lytic life cycle and 0.1% following the lysogenic pathway, infection of an Hfl strain results in 99.9% of infections following the lysogenic pathway and only 0.1% following the lytic pathway.
Infection of an Hfl host with a mixture of recombinant and non-recombinant insertion phage (where insertion inactivates cI - the lambda repressor) results in progeny viruses that are exclusively recombinants. All non-recombinant phage go lysogenic and produce no viral progeny.
The limitation to using lambda insertion vectors is their small insert size capacity. The analysis of transducing phage suggested that as much as 40% of the wild-type genome is dispensible for lytic growth. However viral genomes only 60% of the wild-type length aren't packaged into viable phage particles. Therefore, in order to utilize the full carrying capacity of the lambda vector,
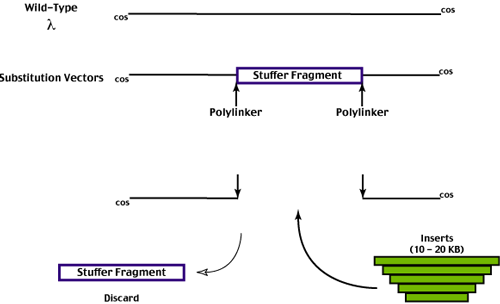
substitution phage vectors were developed.
In order to maintain the vector as a viral stock it must replicate efficiently. To take advantage of the full carrying capacity (vector constitutes 60 % of recombinant genome length, the insert 40 - 45%),
the vector sequence must carry a 'stuffer' fragment which can be easily removed and discarded.
This stuffer is replaced by the foreign DNA fragments we wish to insert.
The foreign DNA fragments substitute for the stuffer fragment.
Substitution vectors (10 - 20 KB) carry larger inserts than insertion vectors (0 - 10 KB).
Selection is less of an issue with substitution vectors. The removal of the stuffer fragment leaves a viral genome that cannot be efficiently packaged, so non-recombinant phage are (theoretically at least) inviable. Of course, it is difficult to completely remove the stuffer fragment.
The selection system used to selectively replicate recombinant phage involves yet another observation made by phage geneticists.
Wild-type lambda phage do not efficiently infect E coli lysogenic for an unrelated bacteriophage P2. Thus, wild-type phage are Sensitive to Ps Interference (spi).
Phage genes responsible for the spi phenotype are carried on the stuffer fragment of the insertion vector. Removal of the stuffer and its replacement by a foreign DNA fragment renders the recombinant phage insensitive to P2 interference.
Therefore we can selectively propage recombinant phage and eliminate nonrecombinants (carrying the stuffer fragment) by growing on an E coli host carrying a P2 lysogen. All of the progeny phage carry foreign DNA inserts because nonrecombinants cannot grow due to the P2 interference.
go to
Library Construction